What Is CRISPR-Cas9?
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) consists of repeating DNA sequences in bacteria. CRISPR-Cas9 functions like a molecular pair of scissors guided by a Global Positioning System (GPS). These repeats store snippets of viruses that previously attacked the bacteria, acting like a memory to recognise viruses later (Martín-Valmaseda et al., 2023).
Cas9 is a protein that works as molecular scissors, cutting DNA precisely. A guide RNA acts as GPS, directing Cas9 to exact DNA locations. In nature, this system protects bacteria from viruses. Scientists adapted it to edit genes in humans, plants, and even insects. Changing the guide RNA allows Cas9 to target almost any gene, making genome engineering programmable and versatile (Khlidj, 2023).
How CRISPR-Cas9 Is Changing Medicine
CRISPR-Cas9 enables precise gene therapy (Li et al., 2023). For instance, mutations causing sickle cell anaemia or muscular dystrophy can be corrected by delivering Cas9 and guide RNA into patient cells (Sharma et al.,2023; Olson, 2021). This approach targets DNA at its source rather than treating symptoms.
The technology also shows promise in preventing inherited diseases (Abdelnour et al., 2021). Editing embryos could prevent certain genetic disorders from passing to future generations. However, such use raises ethical questions owing to permanent effects on the germline (Krekora-Zając, 2020).
CRISPR-Cas9 in Cancer Therapy: Mechanisms and Applications
CRISPR-Cas9 has revolutionised cancer research by enabling precise gene editing. It allows targeted knockout of oncogenes or repair of tumour suppressor genes, directly affecting cancer cell proliferation and survival. By inducing double-strand breaks in DNA, CRISPR can disrupt harmful genes or insert beneficial sequences. In addition to basic research, it supports cancer immunotherapy. For instance, T cells can be engineered with CRISPR to better recognise and attack tumour cells, leading to significant clinical responses in certain leukaemia and lymphoma patients (Liu et al., 2023; Xu et al., 2021).
Personalised and Targeted Cancer Treatment
CRISPR-Cas9 facilitates personalised therapy through a combination with next-generation sequencing. This approach identifies patient-specific mutations, enabling direct targeting of tumour-driving genes. Clinical studies have applied CRISPR to cancers such as glioblastoma, breast cancer, and pancreatic cancer, improving therapeutic outcomes while reducing off-target effects (Selvakumar et al., 2022; Rabaan et al., 2023). Moreover, CRISPR shows promise in overcoming drug resistance by editing genes responsible for chemotherapy failure, restoring drug sensitivity in resistant cancer cells (Vaghari-Tabari et al., 2022).
CRISPR-Cas9 in Blood Disorders
CRISPR-Cas9 provides a precise method to treat inherited blood disorders such as sickle cell disease and β-thalassemia. Scientists extract patients’ hematopoietic stem cells and use CRISPR-Cas9 to correct faulty genes or activate fetal haemoglobin (Levesque & Bauer, 2025). In sickle cell disease, turning on fetal haemoglobin reduces red blood cell sickling, lowers transfusion needs, and improves overall health (Park & Bao, 2021). Early trials showed patients producing normal haemoglobin with lasting benefits.
Similarly, in β-thalassemia, CRISPR boosts functional haemoglobin production, enabling patients to reduce or eliminate transfusions (Meisel, 2021). Therapies such as exa-cel demonstrate CRISPR’s potential as a practical treatment, not just a research tool (Sharma et al., 2023). Corrected stem cells continue producing healthy blood cells, providing long-term improvements and offering hope for other genetic blood disorders.
CRISPR-Cas9 in Eye Diseases
CRISPR-Cas9 offers a promising approach to treating inherited and acquired eye diseases. Researchers target specific faulty genes in retinal cells to restore or preserve vision (Sundaresan et al., 2023). For example, in inherited retinal dystrophies, CRISPR corrects mutations in photoreceptor cells, preventing degeneration and improving retinal function (Chien et al., 2022). Subretinal delivery of CRISPR components allows precise editing without affecting other tissues (Hu et al., 2023).
CRISPR also targets eye conditions caused by abnormal blood vessel growth, such as age-related macular degeneration. Editing genes like Vascular Endothelial Growth Factor A (VEGFA) reduces harmful neovascularisation and protects vision (Sundaresan et al., 2023). VEGFA is a protein that promotes the growth of new blood vessels by stimulating angiogenesis, increasing vessel permeability, and supporting the survival of endothelial cells. Overall, CRISPR-Cas9 demonstrates clinical potential by providing long-term correction, localised delivery, and minimal side effects, highlighting its role as a transformative therapy for ocular diseases (Gowda et al., 2025).
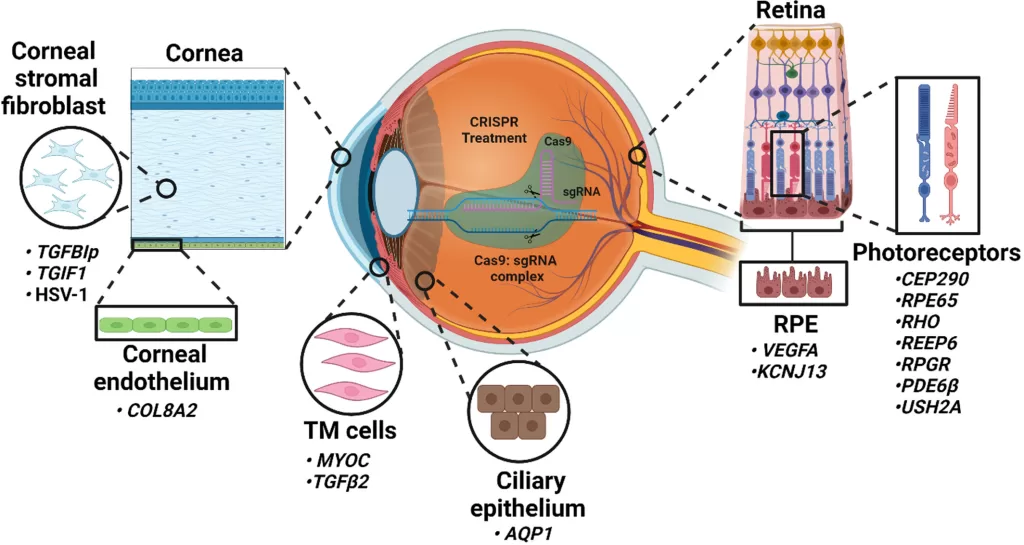
Source: FEBSPRESS Sundaresan et al., 2023
CRISPR-Cas9: A New Hope for HIV Therapy
CRISPR-Cas9 can target HIV-1 genes and host co-receptors, reducing viral replication in cells and animal models (Bhowmik & Chaubey, 2022; Li et al., 2022). It also edits host factors that maintain viral latency, potentially preventing reactivation (Cisneros et al., 2022). Despite challenges like delivery and off-target effects, CRISPR-Cas9 offers a promising path toward long-term HIV control (Chung et al., 2020; Herskovitz et al., 2021).
CRISPR-Cas9: Transforming Liver Disease Therapy
CRISPR-Cas9 technology has rapidly advanced from feasibility studies to potential clinical applications in liver diseases. Initially used to model genetic liver disorders, it now enables precise correction of mutations in metabolic liver diseases, including glycogen storage disorders and Pulmonary Arterial Hypertension (Pah)-related defects (Rutten et al., 2021; Sharma et al., 2021). Moreover, CRISPR-mediated activation of protective pathways, such as Nuclear factor erythroid 2-related factor 2 (NRF2), offers new therapeutic strategies for non-alcoholic fatty liver disease (Li et al., 2023).
NRF2 is a protein that acts as a master regulator of the body’s antioxidant response by activating genes that protect cells from oxidative stress, toxins, and inflammation. Innovations like exosome-mediated Cas9 delivery and liver-specific nanosystems further enhance targeted editing, increasing efficacy while reducing off-target effects (Wan et al., 2022; Xu et al., 2022). Overall, these breakthroughs highlight the transformative potential of CRISPR-Cas9 in both rare and common liver disorders, bridging basic research and translational therapy (Trevisan et al., 2020; Gibson et al., 2025).
Source: Molecular therapy Sharma et al,2021
Feeding the Future: CRISPR in Agriculture and Food Security
CRISPR-Cas9 in Agriculture
CRISPR helps develop crops that resist diseases, tolerate drought, or improve nutrition. For example, tomatoes that remain fresh longer and drought-tolerant rice have been developed using this technology (Shelake et al., 2022). These advances strengthen food security and support sustainable agriculture.
Gene editing also allows precise control over fruit crops, speeding up breeding programmes and reducing reliance on chemical pesticides (Martín-Valmaseda et al., 2023).
Applications in Crop Improvement
CRISPR-Cas9 in Agriculture: Enhancing Crop Yield and Quality
CRISPR-Cas9 has emerged as a transformative tool in modern agriculture, enabling precise genome editing to improve crop yield, quality, and stress resilience. By targeting key genes, researchers have enhanced drought tolerance, disease resistance, and nutrient efficiency across major crops such as rice and maize (Sami et al., 2021; Gillani et al., 2021). Beyond single-trait improvements, CRISPR facilitates hybrid breeding, crop domestication, and multi-trait enhancement, bridging conventional breeding and molecular biotechnology (Rao & Wang, 2021; Liu et al., 2021). These innovations, combined with precision agriculture techniques, promise sustainable productivity gains to address global food security challenges (Chovatiya et al., 2024).
CRISPR-Cas9 in Agriculture: Disease Resistance
CRISPR-Cas9 has revolutionised plant disease management by enabling precise genome edits that enhance resistance to pathogens. Researchers have successfully targeted susceptibility (S) genes and introduced resistance (R) genes in crops, reducing vulnerability to bacterial, viral, and fungal infections (Tyagi et al., 2020; Faizal et al., 2024). These innovations not only lower pre- and post-harvest losses but also provide a sustainable alternative to chemical pesticides, aligning with eco-friendly and regulatory-compliant agricultural practices (Krishna et al., 2022; Das et al., 2023).
CRISPR-Cas9 for Insect Pest Management
Insect pests significantly threaten global crop productivity, and CRISPR-Cas9 offers precise solutions for their control. By editing pest genomes or modifying plant defence pathways, CRISPR enables targeted resistance against major agricultural pests, such as the fall armyworm (Singh et al., 2022; Gouda et al., 2024). The technology supports integrated pest management strategies, minimises chemical pesticide use, and can sustainably enhance crop protection under diverse field conditions (Akbar et al., 2023; Komal et al., 2023).
CRISPR-Cas9 Gene Drives in Vector-Borne Diseases
CRISPR-Cas9 gene drive technology offers a powerful way to control vector-borne infections. By biasing inheritance, gene drives spread traits such as infertility or pathogen resistance throughout insect populations faster than natural selection (Rostami, 2020; Hillary & Ceasar, 2021). This strategy targets disease vectors like mosquitoes, which transmit malaria, dengue, and Zika.
Example: Malaria Control with Mosquito Editing
A well-studied case is the malaria vector Anopheles gambiae. Scientists used CRISPR-Cas9 to disrupt genes crucial for mosquito reproduction, leading to reduced populations (Singh & Das, 2020). Similarly, CRISPR can introduce resistance genes that block Plasmodium transmission in mosquitoes, effectively lowering malaria spread (Harvey-Samuel et al., 2023).
Impact on Vector-Borne Disease Management
By controlling vectors instead of only treating infections, CRISPR provides long-term, eco-friendly disease control. This approach reduces reliance on insecticides, minimises resistance development, and has the potential to eradicate diseases like malaria, dengue, and West Nile virus (Raban et al., 2022; Purusothaman et al., 2021).
Fighting Diseases in the Wild
CRISPR can reduce populations of disease-carrying species. Using gene drives, Cas9 spreads a genetic change quickly, for instance, in mosquitoes to combat malaria. This method provides an alternative to chemical-based control while targeting the root of disease transmission (Zhao et al., 2024; Adnyana et al.,2024; Yadav et al., 2024).
Risks and Ethical Questions
Despite its potential, CRISPR carries off-target risks, meaning Cas9 might cut unintended DNA sequences. These mistakes could lead to unknown effects. Editing embryos is particularly controversial because changes are permanent and affect future generations. Ethical discussions emphasise careful regulation and monitoring before widespread use (Lorenzo et al., 2022).
Why CRISPR-Cas9 Matters
CRISPR-Cas9 represents a revolutionary tool in genome engineering, reshaping medicine, agriculture, and environmental management. With responsible research and ethical oversight, this technology offers unprecedented solutions for genetic diseases, crop improvement, and disease control (Horodecka & Düchler, 2021).
Revolutionising Medicine: CRISPR-Cas9 in Genetic and Infectious Disease Treatment
CRISPR-Cas9 is transforming modern medicine because it offers precise and efficient genome editing. Initially, it was mainly used for basic research; however, it is now advancing into clinical applications (Sharma et al., 2021). Moreover, this technology can directly target faulty genes, which makes it especially promising for treating hereditary diseases such as sickle cell anaemia and Leber congenital amaurosis (Kan & Doudna, 2022). Consequently, patients may experience long-term cures rather than temporary treatments.
In addition, CRISPR-Cas9 plays a vital role in combating infectious diseases. For example, researchers have successfully targeted viral genomes of HIV and hepatitis B, thereby blocking replication and reducing infection (Lin et al., 2021). Furthermore, nanoparticle delivery systems are enhancing it’s efficiency in reaching infected tissues (Dubey et al., 2022). Therefore, CRISPR-based therapies may complement or even replace traditional antivirals in the future.
Overall, CRISPR-Cas9 not only bridges genetic and infectious disease therapies but also accelerates the shift toward personalised medicine. As a result, it stands as a revolutionary tool with the potential to redefine disease treatment worldwide (Binnie et al., 2021).
Conclusion and Call to Action (CTA)
CRISPR-Cas9 is no longer just a laboratory tool; it is reshaping medicine, agriculture, and global health. From correcting genetic blood disorders to designing resilient crops, this technology demonstrates unmatched potential to improve human life and ensure food security (Sharma et al., 2021; Shelake et al., 2022). Moreover, its use in fighting infectious diseases and controlling vectors like malaria-transmitting mosquitoes highlights its broad societal impact (Harvey-Samuel et al., 2023).
However, with great power comes great responsibility. Off-target effects, ethical debates on germline editing, and ecological risks demand cautious and transparent use (Lorenzo et al., 2022). Responsible regulation, global collaboration, and inclusive public dialogue must accompany scientific progress.
The future of CRISPR-Cas9 depends on collective action. Researchers must push innovation while ensuring safety, policymakers must establish clear frameworks, and society must remain engaged in shaping its applications. If developed responsibly, CRISPR can revolutionise healthcare, agriculture, and disease control, offering real solutions to some of the world’s greatest challenges.
Now is the time to invest, regulate, and collaborate so that CRISPR-Cas9 becomes not just a scientific breakthrough, but a global tool for human progress.
Achieving THRIVE goals
CRISPR-Cas9 has moved from experimental science to real-world impact. It holds the power to correct genetic disorders, revolutionise cancer therapy, improve vision in patients with certain eye diseases, and engineer resilient crops (Sharma et al., 2021; Shelake et al., 2022). These advances align closely with the United Nations’ Sustainable Development Goals (SDGs), particularly SDG3 (Good Health and Well-Being) and SDG2 (Zero Hunger). By strengthening health systems and food security, CRISPR offers tools to tackle pressing global challenges.
Yet, despite this potential, significant shortcomings remain in meeting these SDGs. Many CRISPR-based therapies remain costly and inaccessible, especially in low- and middle-income countries. This inequity undermines progress toward SDG10 (Reduced Inequalities). Moreover, ethical debates on germline editing and ecological risks from gene drives highlight the lack of strong global governance frameworks (Lorenzo et al., 2022). Without inclusive regulation and wider access, CRISPR may widen scientific and social divides rather than bridge them.
The way forward requires investment in safe research, international collaboration, and policies that prioritise both equity and ethics. Scientists, policymakers, and society must work together to ensure CRISPR becomes a tool for global good, not just a privilege for a few. Responsible development can transform SDG aspirations into tangible outcomes, bringing healthier lives and sustainable food systems within reach for all.
A Thrivable Framework
1. Systemic Holistic Model
CRISPR-Cas9 transcends medicine, agriculture, and environmental management, showing how one technology can affect multiple interconnected systems. For instance, it improves human health by correcting genetic diseases, strengthens food systems through crop resilience, and supports ecosystem health by reducing chemical pesticide use. This holistic impact reflects the interconnectedness emphasised by the THRIVE Framework through the System Holistic Model.
2. Complex Wicked Problems
Global health crises, food insecurity, and climate-driven disease spread are Complex Wicked Problems with no simple solutions. CRISPR offers innovative pathways such as gene drives against malaria mosquitoes or engineering drought-resistant crops but it also raises ethical, ecological, and governance challenges. This complexity directly aligns with THRIVE’s view of navigating wicked, multi-dimensional challenges.
3. Science-Based Targets
CRISPR applications contribute to measurable outcomes that align with SDG targets, such as reducing hunger (SDG2) or improving health (SDG3). For example, CRISPR-edited crops reduce losses from pests and climate stress, while therapies like exa-cel target measurable reductions in sickle cell anaemia symptoms. This shows how science-driven, evidence-based progress matches the THRIVE Framework’s emphasis on measurable transformation through Science-Based Targets.
4. Values-Based Innovation
CRISPR’s future depends not only on technical success but also on aligning with ethical values such as fairness, equity, and long-term sustainability. Debates around germline editing, accessibility of therapies, and ecological risks highlight the need for value-driven governance. This resonates with THRIVE’s call for Value-Based Innovation.
To learn more about our work, visit our website and explore how we’re making a difference. You can also follow our informative blog and podcast series, where you can dive deeper into the latest sustainability topics. Join our live webinars, where expert guests from various fields share valuable insights and answer your questions in real time. Sign up for our newsletter to stay up-to-date with upcoming events, fresh content, and exclusive opportunities. Watch our YouTube videos, explore our journal and whitepapers, or connect with us on LinkedIn.
Why trust us?
At THRIVE Project, we’re all about facts that matter—and a future that flourishes. Our team of researchers, writers, and thrivability experts dig deep into the science so you don’t have to. Everything we publish is based on credible sources, double-checked for accuracy, and written with one goal in mind: helping you make sense of the world and how to improve it. We’re independent, non-profit, and here to spark real change with knowledge you can count on. Find out more about our team.
– THRIVE Project























