what is a Methane Hydrate?
Ocean methane hydrates are white, icelike solids that consist of methane and water. They are an untapped potential future energy source.
Ocean methane hydrate deposits are a kind of shortcut fossil fuel. Methane gas is primarily formed by microorganisms that live in the deep sediment layers and slowly convert organic substances into methane.
Vast amounts of ocean methane hydrate are stored in sediment deposits on the continental slopes. The total global amount of methane carbon bound up in these hydrate deposits is in the order of 1000-5000 giga-tonnes. This is approximately 100 to 500 times more carbon than the annually amount released into the atmosphere by the burning of fossil fuels.
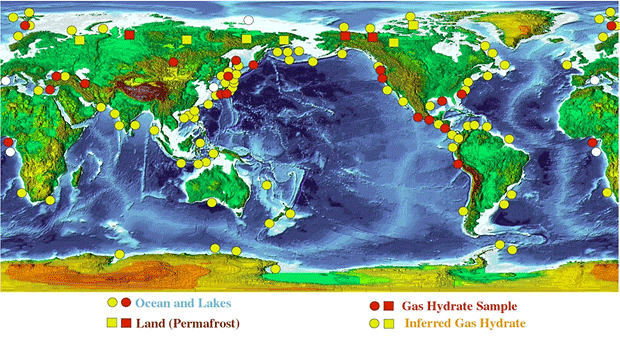
However, Ocean Methane hydrates have recently become a topic of intense discussion within the context of climate change. Methane, which itself acts as a strong greenhouse gas, does not escape directly out of the sea. It is convert into CO2 first. But the formation and release of carbon dioxide are considerable. An additional problem is that the oxygen in seawater is consume through the formation of carbon dioxide.
Global Warming and Methane hydrates
Methane hydrates only form under very specific conditions. Furthermore it could affect the stability of gas hydrates.
There are indications in the history of the Earth suggesting that climatic changes in the past could have led to the destabilization of methane hydrates. Thus to the release of methane.
The issue is highly topical. Scientists are concerned with predicting the possible impacts of a temperature increase on the present deposits of methane hydrate.
Furthermore, Methane is a potent greenhouse gas, around 20 times more effective per molecule than carbon dioxide. An increased release from the ocean into the atmosphere could further intensify the greenhouse effect.
A wide variety of mathematical models are used to predict the consequences of global warming. The results of the simulations are likewise very variable.
It is therefore difficult to precisely evaluate the consequences of global warming for the gas hydrate deposits. Not least of all due to the large differences in the calculations of the size of the present-day gas hydrate deposits.
Some scientists raise the alarm that large quantities of methane (CH4) might be liberat by widespread destabilisation of climate-sensitive gas hydrate deposits trap in marine and permafrost-associated sediments.
Even if only a fraction of the liberated CH4 were to reach the atmosphere, the potency of CH4 as a greenhouse gas (GHG) and the persistence of its oxidative product (CO2) heightened concerns that gas hydrate dissociation. It could represent a slow tipping point for Earth’s contemporary period of climate change.
Why marine life is on high risk?
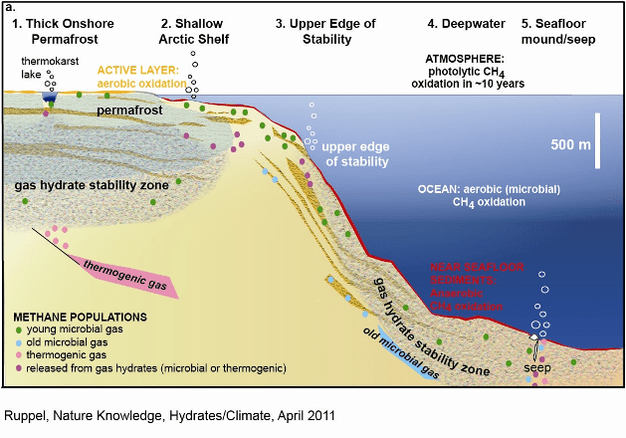
Caps of frozen CO2 or methane, called hydrates that contain the potent greenhouse gases, keeping them from escaping into the ocean and atmosphere.
But the ocean is warming as carbon emissions continue to rise. Scientists say the temperature of the seawater surrounding some hydrate caps is within a few degrees of dissolving them. That will cause huge disturbed marine life environment.
The oceans absorb a third of humanity’s carbon dioxide emissions. Also, 90 percent of the excess heat generated by increased greenhouse gas emissions.
Additionally, it’s the largest carbon sink on the planet. If warming seas melt hydrate caps, there’s a danger that the oceans will become big carbon emitters instead, with grave consequences for climate change and sea level rise.

Conclusion
It is unlikely that we will see significant worldwide gas production from hydrates for the next 30 to 50 years. However, in certain parts of the world characterized by unique economic and/or political motivations. Gas hydrates may become a critical sustainable source of natural gas within the foreseeable future, possibly in the next five to ten years.
Visit THRIVE Project for more information on how you can investigate change towards a more sustainable and thrivable future for all life on Earth.























