Introduction
The rising level of anthropogenic carbon dioxide in the atmosphere has disastrous effects on the climate and Earth’s largest ecosystem, the ocean. 30% of current carbon dioxide (CO2) emissions in the atmosphere are absorbed by the oceans (Shadwick et al., 2023). Thus, substantially moderates the effects of climate change globally. However, it causes a significant decrease in pH levels, and the process is known as ocean acidification. The impacts of ocean acidification threaten to alter the abundance and distribution of life throughout the ocean. Consequently, it affects the livelihoods of millions of people who rely on the ocean.
What is ocean acidification?
Change in Ocean acidity
Carbon dioxide in the atmosphere dissolves into seawater. Water and CO2 combine to form carbonic acid (H2CO3), a weak acid that dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3–). As CO2 levels in the atmosphere increase, they substantially generate more H+ ions in seawater and reduce the ocean pH. The average pH of the ocean has decreased from 8.2 to 8.1 since pre-industrial times. Though this overall change in pH appears small (0.1 pH units over the past 150 years), its impact is enormous. Each decrease of 0.1 pH unit is a ten-fold increase in acidity, indicating 30% more acidity compared to pre-industrial times. According to the Intergovernmental Panel on Climate Change (IPCC)’s projected pathway RCP 8.5 (a potential future global warming scenario if we continue business as usual), oceanic pH will be 7.95 by 2045 (Fig. 1).
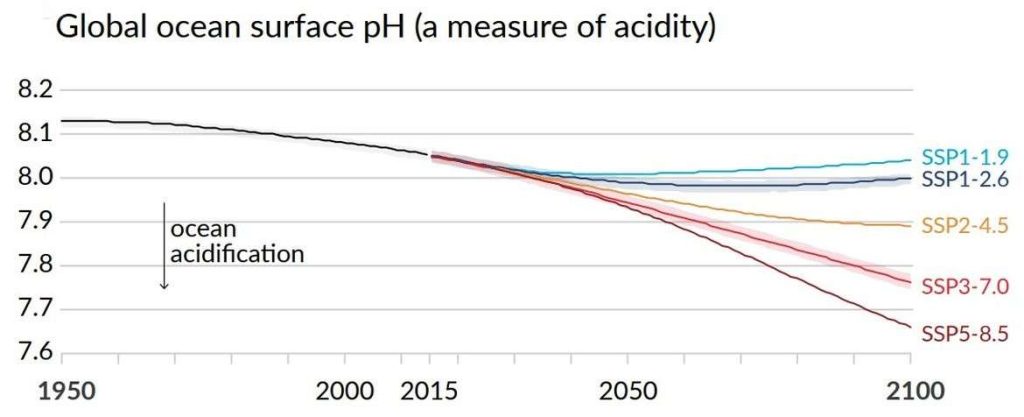
Figure. 1: The graph shows model scenarios with intermediate to high GHG emissions in yellow, red, and maroon lines. The light blue line holds global warming to about 1.5 degrees Celsius (°C), in line with the goals of the Paris Agreement; the dark blue line holds global warming to beneath 2°C.
Source: IPCC Summary for Policymakers
As ocean acidification increases, available carbonate ions (CO32-) bond with excess hydrogen (Fig. 2). This results in fewer carbonate ions for calcifying organisms to build and maintain their shells, skeletons, and other calcium carbonate structures. If the pH gets low, these shells and skeletons can even begin to dissolve. It particularly affects aragonite, the less crystalline CaCO3 that makes corals and shells.
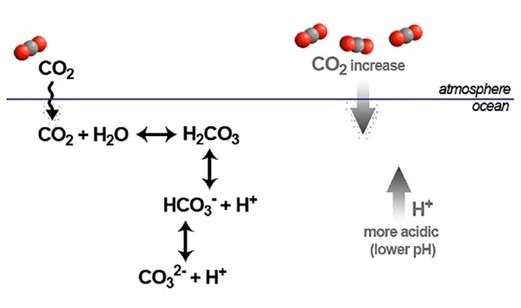
Figure. 2: Schematic representation of the CO2 air-sea exchange and the basic equilibriums of the marine carbonate system in seawater.
Source: Perretti et al., 2018
Impacts of Ocean Acidification
Ocean acidification significantly impacts the ability of calcifiers (Fig. 3) to build their skeletons or shells and alters the sensory abilities of some species. The major groups of calcifying plankton are coccolithophorids, foraminifers, and pteropods. All of them play an important role in the food web. However, pteropods are especially important in high-latitude oceans (Hunt et al., 2008). In benthic ecosystems (ecological systems that exist on or within the seabed), key species such as sea urchins and heart urchins are vulnerable (Miles et al., 2007). Brittlestars are also affected (Wood et al., 2008). Economically valuable species like oysters and mussels will experience seasonal impacts as pH and carbonate ion levels decline (Gazeau et al., 2007).
“Ecosystem engineers” like cold-water and tropical corals are highly sensitive to decreasing aragonite saturation. The reef structures formed by corals provide critical fish habitats and nursing grounds for many organisms (Guinotte et al., 2006). Tropical corals thrive in warm, sunlit waters with high carbonate saturation (Kleypas et al., 2001). They support high biodiversity and provide an essential protein source for many people through fisheries.

Figure. 3: Calcifiers impacted by ocean acidification from top left to bottom right: pteropod, benthic foraminifer, coccolithophore, blue mussel, sea urchin, brittle star, tropical coral, coralline algae, and cold water coral.
Source: Turley et al., 2010
Overall, ocean acidification potentially disrupts the marine food web. Lower sea water pH also affects ocean organisms composed of magnesium calcite. Silica-based diatoms are also sensitive to ocean acidification. Carbonate shells start to dissolve at pH 8.04 and the process is essentially completed at pH 7.95. Lower pH already started affecting marine calcifiers. Some organisms may survive this stressor or evolve to cope with lower pH. But their reproduction will be seriously compromised, and they will be stressed and predisposed to infection.
Threats to Pteropods; a Calcifying Species
Pteropods are shelled, free-swimming marine snails. Research shows a significant reduction in their ability to form CaCO3 shells at a lower pH. The photographs (Fig. 4) show what happens to a pteropod, exposed to ocean water adjusted to a pH of 7.8. This pH is projected towards the year 2100 under high emission scenarios (Fig. 1). The experiment evidenced that the shell dissolves after approximately 45 days. Though research suggests the ability of pteropods to repair and maintain their shells, despite progressive loss, they are vulnerable to ocean acidification (Peck et al., 2018).
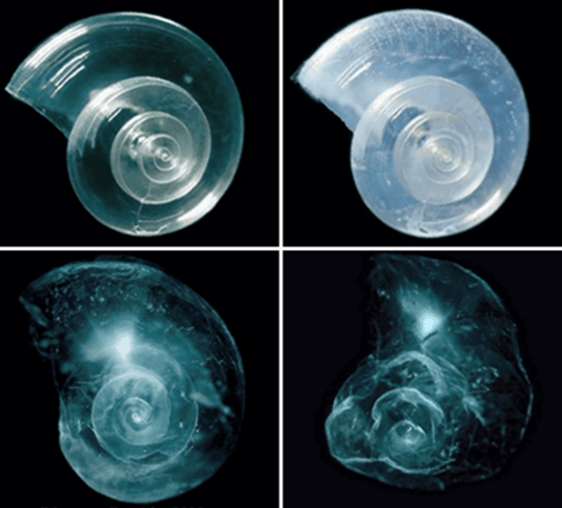
Figure. 4: Panels from the top represent 0 days, 15 days, 30 days and 45 days, respectively. Source: NOAA PMEL
The tiny pteropods are the primary food source for many marine animals, including herring, mackerel, tuna, walleye pollock, squids, large shrimp, and whales (aoan.aoos.org). The decline of such marine calcifiers owing to ocean acidification can disrupt the entire marine food web. The extinction of skeleton-based marine creatures is expected by the end of this century if acidification continues unabated (www.aims.gov.au).
Impact of Ocean Acidification on Coral Reefs
Ocean acidification is likely to cause major shifts in marine ecosystems and food webs, including the loss of most coral reefs globally. Coral reefs occupy only 0.1% of the ocean’s bottom (www.fisheries.noaa.gov). Yet they are home to more than a quarter of all marine life, including crustaceans, reptiles, seaweeds, bacteria, fungi, and over 4000 species of fish (Fisher et al., 2015). These ecosystems sustain coastal economies, and the benefits flow to over 500 million people (www.unep.org). Coastal economies depend on reef-related tourism to develop novel pharmaceuticals and supply food for hundreds of millions of people (World Wildlife Fund, 2020). The asset value of coral reefs has been estimated to be USD $1 trillion (Hoegh-Guldberg, 2015), with the economic value of goods and services from coral reefs exceeding USD $375 billion annually.
According to the report Reefs at Risk Revisited, 75% of the world’s coral reefs are at risk from local and global stresses. 50% of the world’s reefs have already died in the last 30 years. More than 90% of the remaining reefs are projected to die by 2050 (Independent, 13 March 2017). The decreased production of calcium carbonate, along with increased carbonate dissolution, results in slower growth rates and weaker coral skeletons (library.gbrmpa.gov.au). Studies reveal that almost all coral reefs will degrade from their current state, even if global warming remains below 2ºC, and the remaining reef communities will differ in species composition and diversity (IPCC, 2019). It will significantly impact the food security of the global population.
Further Impacts of Acidification
Further consequences of ocean acidification include the reduction in fisheries and tourism, impacts on human health, and decreased coastal protection (coastadapt.com.au). Increased vulnerability stems from the heavy reliance of coastal communities and economies, including Small Island Developing States (SIDS), on coastal and marine resources for sustenance and livelihoods.
The impacts on marine organisms are further exacerbated by other climate change-related stressors, including warming and de-oxygenation, and local factors like land-based pollution, upwelling, and habitat loss (Kroeker et al., 2013). Upwelling events significantly reduce pH, DO, and temperatures in certain regions, increasing the risk of ocean acidification and deoxygenation. However, seagrass meadows may help mitigate the adverse effects of reduced pH and DO during upwelling events through increased photosynthesis in nutrient-rich upwelled waters (www.nairobiconvention.org). Nature has equilibrating mechanisms to balance the impacts it causes, However, immediate action is required to minimise human-induced emissions.
Projected consequences of acidification on marine life
The GOES Project team compiled the ocean acidification and productivity graph that helps to visualise the data presented by the IPCC committee and respected journals. It combines the pH and biodiversity loss and informs the prediction for a pH tipping point (Fig. 5).
As per Dryden et al., 2021, the red line in the graph maps data from peer-reviewed papers to provide a historical pH profile from the 1940s to the present. IPCC data was applied to provide a projected pH profile (RCP 8.5). These data suggest that a pH tipping point will be reached by 2045, beyond which it will not be possible to recover most carbonate-based life forms. At the tipping point, the changes in pH are sufficient to disrupt critical marine processes such as calcium carbonate shell formation and marine food web stability. Though there are debates about this graph, it is worth knowing about this study.
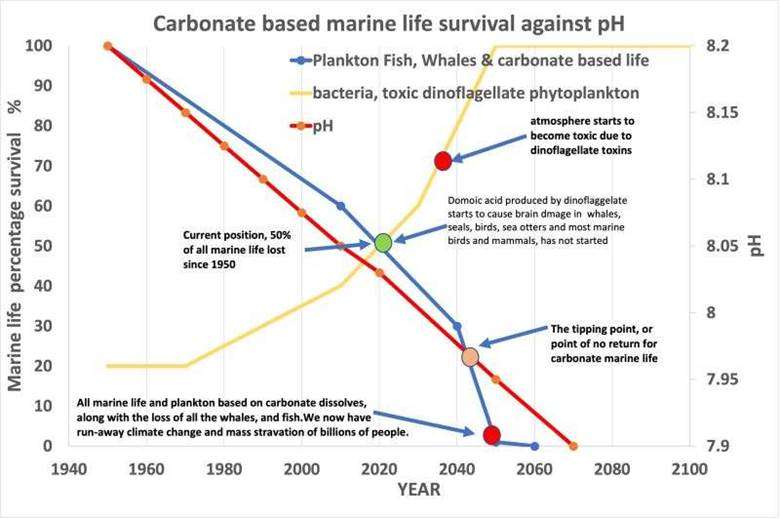
Figure. 5: A graph showing the carbonate-based marine life percentage survival against pH.
Source: LinkedIn post of Dryden
The blue line in the graph represents the reported decline in marine life, reflecting a global decrease of approximately 40% in marine plankton populations since 1950, along with models indicating an annual 1% reduction in phytoplankton. The extrapolation forward from 2020 is based on the BIOACID report. Carbonate-based marine life cannot adapt to the dissolution of calcium carbonate which will lead to their massive disruption after pH tipping point.
Alteration of The food web due to acidification
Acidification alters nutrient cycles and food web dynamics, often creating conditions that favour resilient organisms like bacteria and certain dinoflagellates. Dianoflagellates are single-celled planktonic organisms, but they are more resilient due to their mixotrophic ability to get energy and nutrients in more than one way—usually both photosynthesis and eating other organisms. They also produce DMSP (dimethylsulfoniopropionate) inside their cells when they are stressed as a survival mechanism. The Yellow Line in the graph (Fig. 5) is the prediction for the increased growth of bacteria and dinoflagellates under reduced ocean pH. Domoic acid, a neurotoxin produced by certain dinoflagellates poses significant risks to marine ecosystems, human health, and the economy.
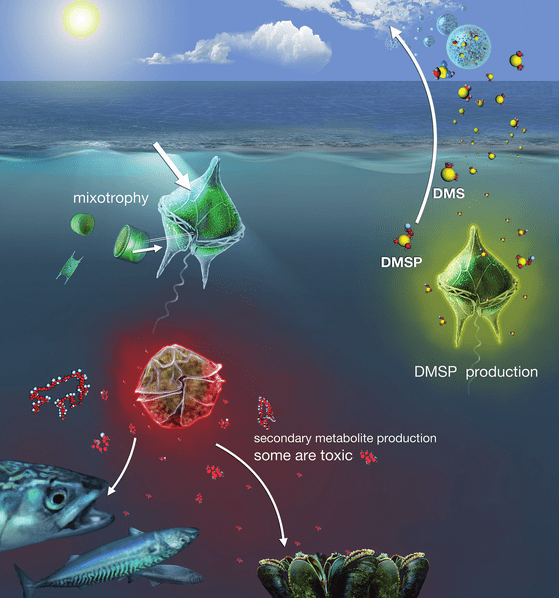
Fig. 6: Some of the major ecological traits of dinoflagellates: free-living planktonic cells, DMSP production, mixotrophy, and the production of toxic/non-toxic metabolites with impacts on other marine life.
Source: Murray et al., 2016
Conclusion and Call to Action (cTA)
Ocean acidification, driven by rising CO₂ emissions, disrupts marine ecosystems. These changes threaten biodiversity, fisheries, and coastal communities reliant on healthy oceans. Immediate action is vital to curb emissions, enhance marine conservation, and promote sustainable practices to protect ocean health and global livelihoods.
If we halt and reverse ocean warming and pollution through global cooperation, we give marine ecosystems a chance to come back from the brink. Major strategies must be implemented urgently and concurrently to mitigate the impacts, such as limiting fossil fuel emissions and building the resilience of tropical marine ecosystems and communities. Concerning climate change, we are fortunate that a political agreement around the next best steps already exists. If carried out properly, the politically motivated Paris Climate Agreement represents the most economically favourable pathway to follow. THRIVE Project advocates for thrivability by supporting aligned organisations and leaders prioritising sustainable futures.
A Thrivable Framework to ACHIeVE UN SUSTAINABLE DEVELOPMENT GOALS
The United Nations’ 2030 Agenda for Sustainable Development addresses this issue through the Sustainable Development Goals (SDGs). SDG14: Life Below Water aims sustainable use of marine resources. Target 14.3 focuses on i) reducing CO₂ emissions (the main driver of acidification), ii) supporting ocean monitoring and research, and iii) strengthening the resilience of marine ecosystems. The focus factors of THRIVE are based on long-term sustainability and responsible innovation, which contribute to achieving SDGs. In the THRIVE Project, ocean governance is a key area of consideration. Context-Based Sustainability Metrics, as emphasised in the THRIVE Framework, help to measure sustainability by measures that encourage maintaining ocean pH. Strong Sustainability emphasises real-world thresholds, promoting decisions that respect natural systems and supporting ecosystem health over short-term fixes. Science-Based Targets promotes circular blue economies and green marine technologies that lower carbon emissions. These strategies further support local adaptation, ocean monitoring, and work towards a healthy ocean.
For further information about climate change, important innovations and environmental footprint, visit THRIVE Project, designed to guide you towards thrivable prosperity. Engage with THRIVE’s clusters and resources, including podcasts, webinars, whitepapers, and the blog. Stay informed via our website and LinkedIn for updates.























